Understanding Metallic Bonding Examples: A Comprehensive Guide
These bonds are formed when metal atoms pool their valence electrons into a "sea of electrons," creating a shared electron cloud that binds the positively charged metal ions. This type of bonding is responsible for the shiny luster, thermal conductivity, and ductility that we associate with metals. Understanding metallic bonding examples helps us appreciate the science behind everyday materials, from the copper wires in our homes to the aluminum foil in our kitchens. In this article, we will explore metallic bonding examples in detail, diving into their characteristics, applications, and real-world significance. Whether you're a student studying chemistry or simply curious about the science behind materials, this guide will provide valuable insights. We'll also address common questions about metallic bonding and examine specific examples that highlight its importance in various industries. By the end of this article, you'll have a clear understanding of how metallic bonds influence the world around us. To ensure you get the most out of this guide, we've organized the content into clear sections. From defining metallic bonding to examining its role in alloys and advanced materials, we'll cover all the essential aspects. Along the way, we'll incorporate metallic bonding examples to make the concepts more relatable. Whether you're looking for practical applications or theoretical explanations, this article has something for everyone. Let’s dive in and uncover the fascinating world of metallic bonding.
Table of Contents
- What is Metallic Bonding?
- How Do Metallic Bonds Form?
- What Are the Properties of Metallic Bonding?
- Examples of Metallic Bonding in Everyday Life
- Why Are Metallic Bonds Important in Alloys?
- How Does Metallic Bonding Affect Materials Science?
- What Are Some Advanced Applications of Metallic Bonding?
- Frequently Asked Questions About Metallic Bonding
What is Metallic Bonding?
Metallic bonding is a type of chemical bond that occurs between metal atoms. Unlike covalent or ionic bonds, metallic bonds involve the pooling of valence electrons into a shared "electron sea." This electron cloud surrounds positively charged metal ions, creating a strong and flexible bond. The unique nature of metallic bonding examples lies in their ability to explain the physical and chemical properties of metals.
One of the defining features of metallic bonding is its non-directional nature. Unlike covalent bonds, which are localized between specific atoms, metallic bonds extend throughout the entire metal lattice. This explains why metals are malleable and ductile. When force is applied, the layers of metal ions can slide past one another without breaking the bond, thanks to the freely moving electrons. This characteristic is evident in metallic bonding examples like gold and copper, which can be hammered into thin sheets or drawn into wires.
Read also:Exploring Adam Savages Children A Glimpse Into Their Lives And Influence
Another key aspect of metallic bonding is its contribution to electrical and thermal conductivity. The free electrons in the electron sea can move easily when an electric field is applied, making metals excellent conductors of electricity. Similarly, these electrons transfer heat efficiently, which is why metals like aluminum and iron are used in cookware and radiators. By examining metallic bonding examples, we can better understand why metals are indispensable in modern technology and industry.
How Metallic Bonds Differ from Other Types of Bonds
To fully grasp metallic bonding, it's important to compare it with other types of chemical bonds. Covalent bonds involve the sharing of electron pairs between atoms, while ionic bonds result from the transfer of electrons to create oppositely charged ions. Metallic bonds, on the other hand, involve a collective sharing of electrons across a lattice of metal ions.
This difference is reflected in the properties of materials. For instance, covalent compounds like diamond are hard but brittle, while ionic compounds like table salt are brittle and non-conductive in solid form. In contrast, metallic bonding examples such as iron and silver exhibit high strength, malleability, and conductivity. These properties make metals uniquely suited for applications ranging from construction to electronics.
How Do Metallic Bonds Form?
The formation of metallic bonds begins with the interaction between metal atoms. Metals typically have few valence electrons, which are loosely held in their outermost shells. When metal atoms come together, these electrons are released into a shared pool, creating a "sea of electrons" that surrounds the positively charged metal ions. This process is a defining characteristic of metallic bonding examples and explains the cohesive nature of metals.
The strength of metallic bonds depends on several factors, including the number of valence electrons and the size of the metal ions. For example, metals with more valence electrons, such as transition metals, tend to form stronger bonds. This is why tungsten, with its high electron density, is incredibly strong and has a high melting point. On the other hand, alkali metals like sodium have fewer valence electrons and weaker bonds, making them softer and more reactive.
Understanding how metallic bonds form also helps us predict the behavior of metals under different conditions. For instance, heating a metal increases the kinetic energy of its atoms, causing the bonds to weaken and eventually break. This explains why metals expand when heated and why they can be melted and reshaped. By studying metallic bonding examples, we gain insights into the fundamental processes that govern material behavior.
Read also:Barry Weiss The Visionary Leader Transforming Industries
What Role Do Electrons Play in Metallic Bonding?
Electrons are the cornerstone of metallic bonding, acting as the "glue" that holds metal atoms together. In metallic bonding examples, the valence electrons are delocalized, meaning they are not tied to any specific atom. Instead, they move freely throughout the metal lattice, creating a dynamic and flexible bond.
This delocalization of electrons has several important implications. First, it allows metals to conduct electricity and heat efficiently. When an electric field is applied, the free electrons move in response, carrying charge through the material. Similarly, the movement of electrons facilitates the transfer of thermal energy, making metals effective heat conductors. This property is evident in metallic bonding examples like copper wiring and aluminum heat sinks.
Second, the mobility of electrons contributes to the malleability and ductility of metals. When stress is applied, the layers of metal ions can slide past one another without breaking the bond, as the electrons continue to hold them together. This is why metals like gold and silver can be shaped into intricate designs without fracturing. By understanding the role of electrons, we can appreciate the versatility and utility of metallic bonds in various applications.
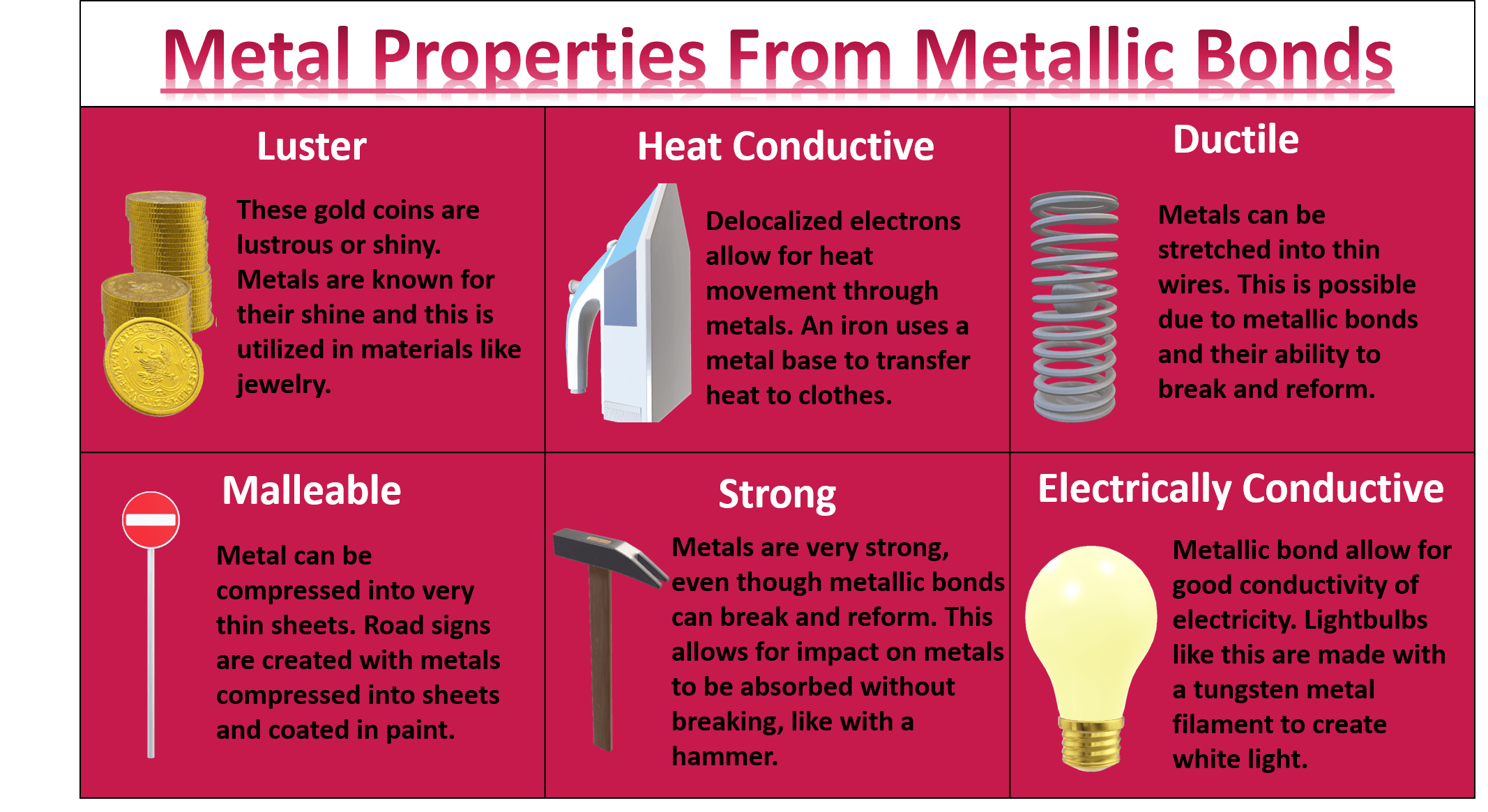
What Are the Properties of Metallic Bonding?
Metallic bonding imparts a unique set of properties to metals, making them indispensable in countless applications. One of the most notable characteristics of metallic bonding examples is their high electrical and thermal conductivity. The free electrons in the electron sea move easily in response to an electric field or heat source, enabling metals to transfer energy efficiently. This property is why metals like copper and silver are widely used in electrical wiring and thermal management systems.
Another key property is malleability and ductility. Metals can be hammered into thin sheets (malleability) or drawn into wires (ductility) without breaking. This is due to the non-directional nature of metallic bonds, which allows layers of metal ions to slide past one another. For instance, gold is so malleable that it can be beaten into sheets just a few atoms thick, a characteristic that has made it valuable in jewelry and electronics. These metallic bonding examples highlight the adaptability of metals in various forms.
Metals also exhibit high tensile strength and durability, thanks to the strong cohesive forces between metal ions and the electron sea. This makes them ideal for structural applications, such as in construction and transportation. Additionally, metals have a characteristic luster, which arises from the interaction of free electrons with light. This shiny appearance is one of the defining features of metallic bonding examples and is often used in decorative and aesthetic applications.
Why Are Metals Good Conductors of Electricity?
The conductivity of metals is one of the most fascinating aspects of metallic bonding examples. Metals are excellent conductors of electricity because their free electrons can move easily when an electric field is applied. Unlike in insulators, where electrons are tightly bound to atoms, the delocalized electrons in metals are free to flow, carrying charge across the material.
This property is particularly evident in metals like copper and aluminum, which are widely used in electrical wiring and circuits. Copper, for instance, has a high density of free electrons, making it one of the best conductors available. Its ability to transfer electricity with minimal resistance ensures efficient energy transmission, reducing losses in power grids and electronic devices.
Thermal conductivity is another related property of metallic bonding examples. Just as free electrons facilitate the flow of electricity, they also transfer heat efficiently. This dual capability is why metals are used in applications like heat sinks, radiators, and cookware. By understanding why metals are good conductors, we can better appreciate their role in modern technology and industry.
Examples of Metallic Bonding in Everyday Life
Metallic bonding examples are all around us, shaping the way we live and interact with the world. One of the most common examples is copper wiring, which is used in homes, offices, and industrial settings. Copper's excellent electrical conductivity, thanks to its strong metallic bonds, ensures efficient energy transfer and minimizes power loss. This makes it an essential component of modern infrastructure.
Another everyday example is aluminum foil, which demonstrates the malleability and ductility of metallic bonding. Aluminum's ability to be rolled into thin sheets without breaking is a direct result of its metallic bonds. This property makes it ideal for food packaging, insulation, and even aerospace applications. Similarly, stainless steel, an alloy of iron, chromium, and nickel, showcases the strength and corrosion resistance imparted by metallic bonding. It is widely used in kitchenware, construction, and medical instruments.
Gold is another striking example of metallic bonding in action. Its resistance to corrosion and tarnishing, combined with its malleability, has made it a favorite in jewelry and electronics. Gold's unique properties are a testament to the versatility of metallic bonding examples, which continue to influence both traditional and cutting-edge industries.
How Does Metallic Bonding Affect Jewelry Design?
In the world of jewelry, metallic bonding examples play a crucial role in determining the durability and aesthetic appeal of materials. Metals like gold, silver, and platinum are prized for their luster and resistance to corrosion, qualities that arise from their metallic bonds. These bonds allow metals to be shaped into intricate designs without losing their structural integrity.
Gold, in particular, is a favorite among jewelers due to its malleability and ductility. It can be hammered into thin sheets or drawn into fine wires, making it ideal for crafting delicate pieces. Similarly, silver's reflective surface and resistance to oxidation make it a popular choice for jewelry and decorative items. By understanding how metallic bonding affects jewelry design, we can appreciate the science behind the beauty of these materials.
Why Are Metallic Bonds Important in Alloys?
Alloys are a testament to the versatility of metallic bonding examples, as they combine the properties of multiple metals to create materials with enhanced characteristics. By mixing metals, scientists and engineers can tailor the properties of alloys to meet specific needs. For instance, steel, an alloy of iron and carbon, is stronger and more durable than pure iron, thanks to the reinforcement provided by metallic bonds.
Another example is bronze, an alloy of copper and tin. The addition of tin improves the strength and hardness of copper, making bronze suitable for tools, sculptures, and architectural elements. Similarly, brass, an alloy of copper and zinc, is valued for its corrosion resistance and golden appearance. These metallic bonding examples demonstrate how alloys leverage the properties of individual metals to create materials with superior performance.
Modern alloys, such as titanium-based and nickel-based superalloys, push the boundaries of what metals can achieve. These materials are used in extreme environments, such as jet engines and spacecraft, where high strength, heat resistance, and corrosion resistance are critical. By understanding the role of metallic bonds in alloys, we can appreciate their importance in advancing technology and industry.
What Are the Advantages of Using Alloys Over Pure Metals?
While pure metals have their own merits, alloys often outperform them in terms of strength, durability, and versatility. One of the primary advantages of alloys is their enhanced mechanical properties. For example, steel is much stronger than pure iron, making it ideal for construction and manufacturing. This improvement is due to the way metallic bonds in alloys distribute stress more effectively.
Another advantage is improved resistance to environmental factors.
Laura Deibel: Unveiling The Inspiring Journey Of A Visionary Leader
Discover The Magic Of Folk Art At The Folk Art Museum New York: A Cultural Treasure Trove
Exploring The Artistry Of Nude Luke Evans: A Comprehensive Guide
Metallic Bonding Transparent Background PNG Cliparts Free, 55 OFF

Metallic Bonding Labelled Diagram