Understanding The Chemical Formula For Hypochlorous Acid: A Comprehensive Guide
Hypochlorous acid, represented by the chemical formula HOCl, is a fascinating compound that plays a crucial role in various fields, from disinfection to healthcare. This weak acid, formed when chlorine dissolves in water, is known for its powerful oxidizing properties. Despite its simplicity, HOCl has proven to be a game-changer in maintaining hygiene and preventing the spread of harmful microorganisms. Its versatility and effectiveness make it a cornerstone of modern sanitation practices.
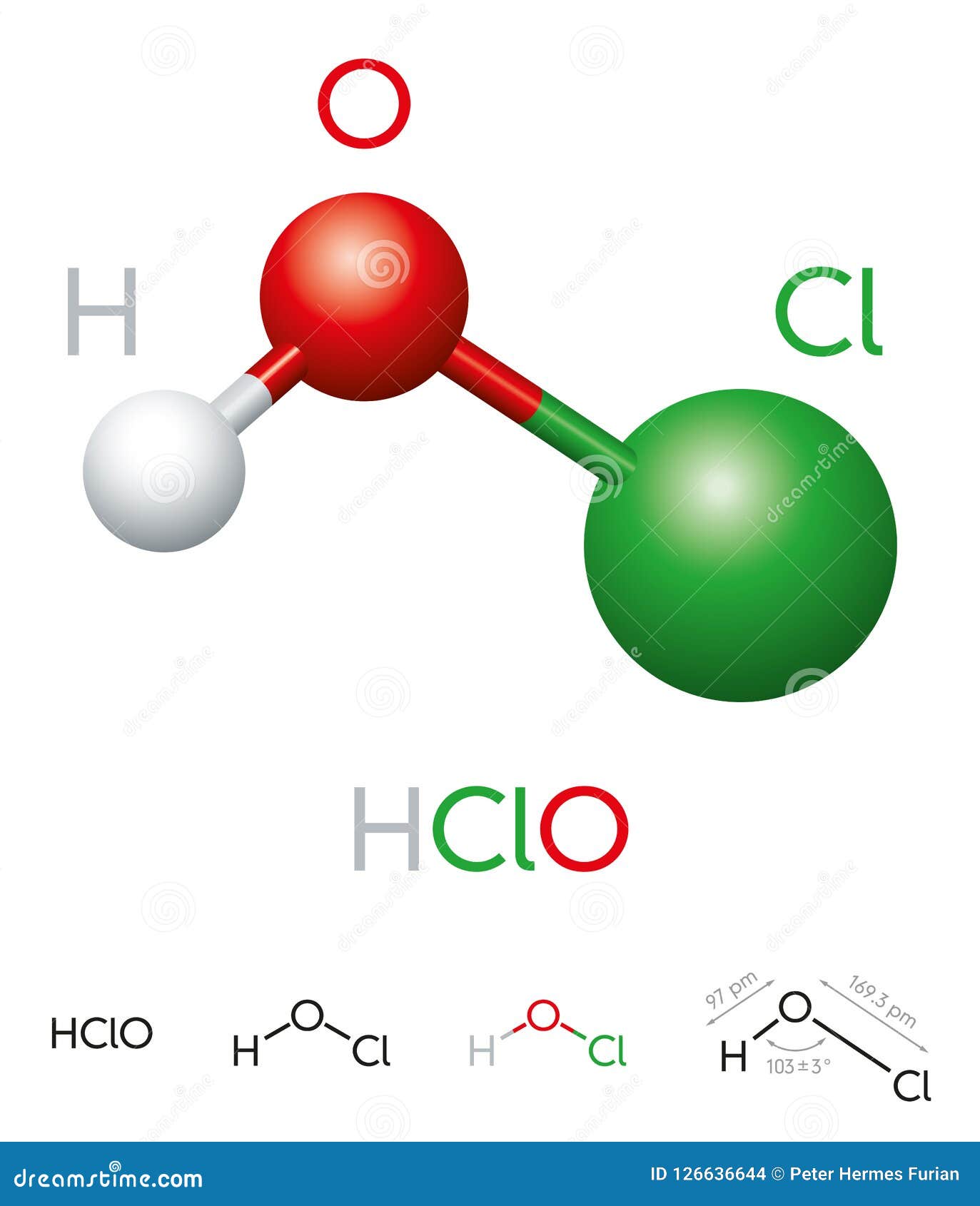
The chemical formula for hypochlorous acid reveals its composition: one hydrogen atom, one oxygen atom, and one chlorine atom. This structure allows HOCl to break down quickly in the environment, making it both effective and eco-friendly. It is widely used in water treatment, wound care, and food safety, demonstrating its broad applicability. With growing concerns about health and hygiene, understanding the role of HOCl has never been more important.
As industries increasingly adopt sustainable and safe solutions, hypochlorous acid has emerged as a preferred choice. Its ability to neutralize pathogens without leaving harmful residues has positioned it as a key player in public health. Whether you're a scientist, healthcare professional, or simply curious about chemistry, exploring the chemical formula for hypochlorous acid offers valuable insights into its mechanisms and benefits. Let’s delve deeper into its properties, uses, and significance.
Read also:Discover The Magic Of Richard Dreyfuss Movies A Journey Through Time
Table of Contents
- What is Hypochlorous Acid (HOCl)?
- How Does HOCl Work as a Disinfectant?
- Applications of Hypochlorous Acid in Daily Life
- Is Hypochlorous Acid Safe for Everyday Use?
- What is the Environmental Impact of HOCl?
- Why is HOCl Preferred Over Other Disinfectants?
- How to Use Hypochlorous Acid Effectively?
- Frequently Asked Questions About Hypochlorous Acid
What is Hypochlorous Acid (HOCl)?
Hypochlorous acid, with the chemical formula HOCl, is a naturally occurring compound that forms when chlorine dissolves in water. This weak acid is highly reactive and is known for its ability to kill bacteria, viruses, and fungi. It is produced in the human body by white blood cells as part of the immune response, making it an essential component of our natural defense mechanisms. Despite its potency, HOCl is gentle enough to be used in sensitive applications like wound care and food sanitation.
Understanding the chemical formula for hypochlorous acid provides insight into its unique properties. The molecule consists of one hydrogen atom (H), one oxygen atom (O), and one chlorine atom (Cl). This simple structure allows HOCl to break down rapidly, leaving no harmful residues behind. Its instability is both a strength and a limitation, as it ensures safety but requires careful handling to maintain its effectiveness.
HOCl is often compared to bleach (sodium hypochlorite), but there are key differences. While bleach is a strong base and can be corrosive, hypochlorous acid is a weak acid that is non-irritating and safe for use on skin and surfaces. This distinction makes HOCl a preferred choice for applications where safety and efficacy are paramount. Whether in healthcare, agriculture, or household cleaning, the chemical formula for hypochlorous acid underpins its widespread adoption.
How Does HOCl Work as a Disinfectant?
The effectiveness of hypochlorous acid as a disinfectant lies in its ability to oxidize microbial cells. When HOCl comes into contact with pathogens, it disrupts their cell walls and damages essential proteins and DNA. This process, known as oxidation, renders the microorganisms incapable of reproducing or causing harm. Unlike other disinfectants, HOCl achieves this without the need for high concentrations or harsh chemicals.
The Oxidation Process Explained
The oxidation process begins when HOCl releases chlorine radicals. These highly reactive molecules target the lipids and proteins in bacterial membranes, causing them to break down. For example, the chemical reaction can be represented as:
HOCl + Pathogen → Oxidized Pathogen + Byproducts
Read also:Unblocked Games Your Ultimate Guide To Fun And Learning
This reaction is both rapid and efficient, making HOCl a powerful tool in combating infections and maintaining cleanliness.
How Does HOCl Compare to Other Disinfectants?
Unlike alcohol-based or phenolic disinfectants, hypochlorous acid is non-toxic and biodegradable. It does not produce harmful fumes or residues, making it safer for prolonged use. Additionally, HOCl is effective against a broader range of pathogens, including spores and biofilms, which are often resistant to other cleaning agents. This versatility has led to its adoption in hospitals, schools, and food processing plants worldwide.
Applications of Hypochlorous Acid in Daily Life
The chemical formula for hypochlorous acid, HOCl, has enabled its integration into numerous industries. Its applications span from healthcare to agriculture, showcasing its adaptability and effectiveness. Below are some of the most common uses of HOCl in daily life.
Water Treatment and Purification
One of the primary uses of hypochlorous acid is in water treatment. HOCl is added to drinking water to eliminate harmful bacteria and viruses, ensuring it is safe for consumption. It is also used in swimming pools and wastewater treatment plants to maintain hygiene and prevent the spread of waterborne diseases.
Healthcare and Wound Care
In healthcare settings, HOCl is used as a disinfectant for surfaces and medical equipment. It is also applied to wounds to prevent infections without causing irritation. Its gentle nature makes it suitable for sensitive skin, and it has been shown to accelerate the healing process in chronic wounds.
Is Hypochlorous Acid Safe for Everyday Use?
Yes, hypochlorous acid is considered safe for everyday use when handled properly. Its non-toxic and non-irritating properties make it suitable for a wide range of applications, from household cleaning to personal care. Unlike bleach or ammonia, HOCl does not produce harmful fumes or residues, reducing the risk of adverse reactions.
Why is HOCl Preferred in Sensitive Environments?
HOCl is often used in environments where safety is a priority, such as schools, hospitals, and homes with young children or pets. Its ability to disinfect without causing harm makes it an ideal choice for these settings. Additionally, its rapid breakdown ensures that it does not linger in the environment, further enhancing its safety profile.
What is the Environmental Impact of HOCl?
The environmental impact of hypochlorous acid is minimal compared to other disinfectants. HOCl breaks down into harmless byproducts like water and salt, leaving no toxic residues behind. This makes it an eco-friendly alternative to harsh chemicals that can harm ecosystems and contribute to pollution.
How Does HOCl Contribute to Sustainability?
By reducing the need for synthetic chemicals, HOCl supports sustainable practices in industries like agriculture and healthcare. Its biodegradability and low environmental footprint align with global efforts to minimize the impact of human activities on the planet.
Why is HOCl Preferred Over Other Disinfectants?
The benefits of hypochlorous acid are numerous, making it a preferred choice for disinfection and sanitation. Its effectiveness, safety, and environmental friendliness set it apart from traditional cleaning agents. Below are some of the key advantages of using HOCl:
- Non-toxic and non-irritating
- Effective against a wide range of pathogens
- Biodegradable and eco-friendly
- Suitable for sensitive applications
How to Use Hypochlorous Acid Effectively?
Using hypochlorous acid effectively requires understanding its properties and limitations. Here are some tips for maximizing its benefits:
- Store HOCl solutions in opaque containers to prevent degradation from light.
- Use it within a few days of preparation to ensure maximum effectiveness.
- Apply it to surfaces or wounds as directed by product guidelines.
Frequently Asked Questions About Hypochlorous Acid
What is the chemical formula for hypochlorous acid?
The chemical formula for hypochlorous acid is HOCl. It consists of one hydrogen atom, one oxygen atom, and one chlorine atom.
Is hypochlorous acid safe for skin?
Yes, hypochlorous acid is safe for skin. It is non-irritating and gentle, making it suitable for use in wound care and personal hygiene.
Can hypochlorous acid replace bleach?
Yes, hypochlorous acid can replace bleach in many applications. It is non-toxic, biodegradable, and equally effective against pathogens.
In conclusion, the chemical formula for hypochlorous acid, HOCl, represents a compound with immense potential to improve health and safety. Its unique properties make it a versatile and eco-friendly solution for disinfection and sanitation. By understanding its applications and benefits, we can harness the power of HOCl to create a cleaner, safer world. For more information on hypochlorous acid, visit CDC Guidelines on Disinfectants.
Who Owns MeTV: A Comprehensive Guide To Ownership And Its Impact On Pop Culture
Is Doraemon Real? Exploring The Truth Behind The Iconic Robotic Cat
How Did Escanor Die: The Tragic Story Of The Lion's Sin
HClO Hypochlorous Acid Molecule Model and Chemical Formula Stock Vector

Hypochlorous Acid Hclo Molecule Chemical Structure Stock Illustration