Understanding The Formula For Hypochlorous Acid: A Comprehensive Guide
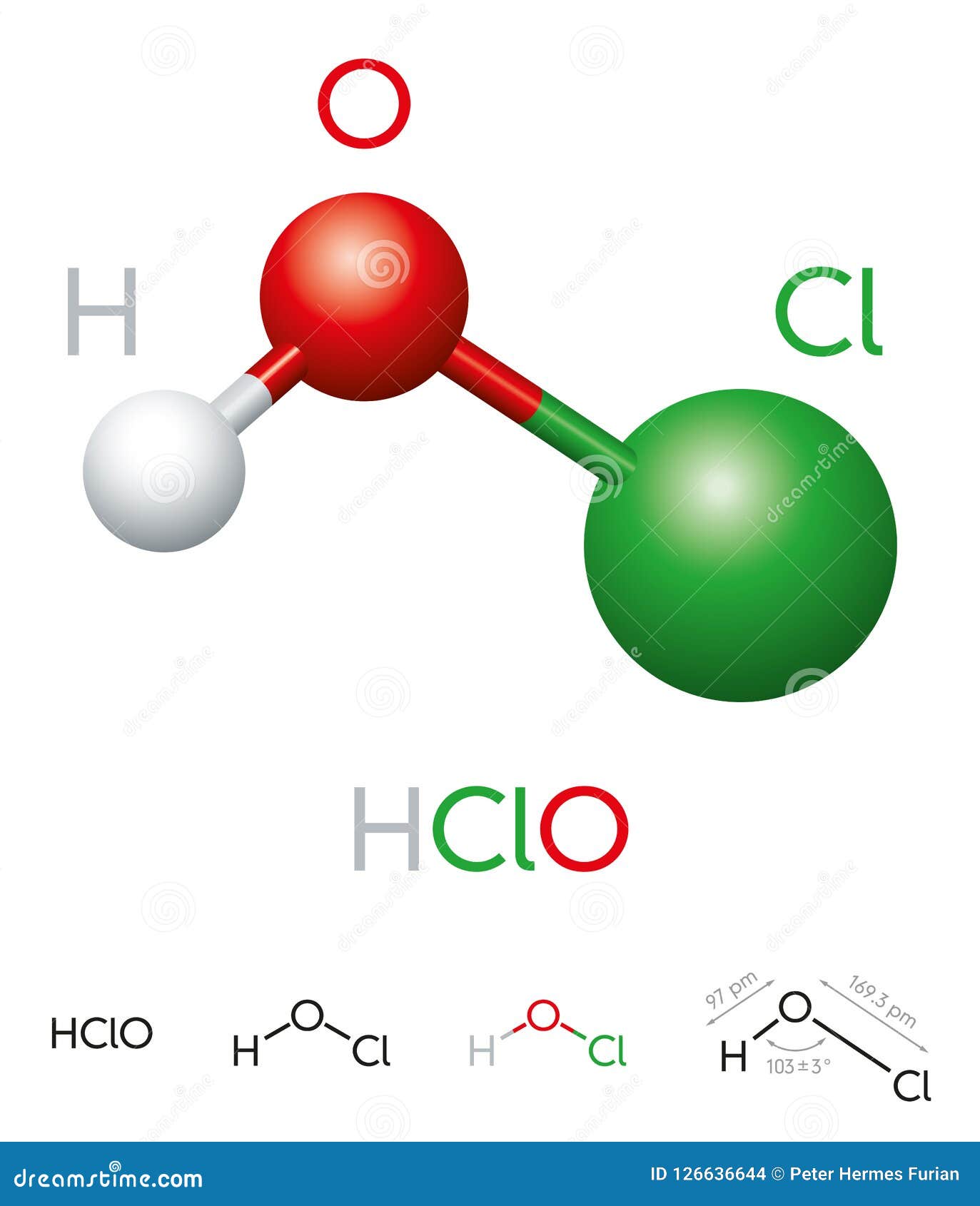
Hypochlorous acid, often represented by the chemical formula HOCl, is a weak acid that plays a pivotal role in various industries, from water treatment to healthcare. Its importance cannot be overstated, as it serves as a powerful oxidizing agent and disinfectant. Despite its potency, hypochlorous acid is gentle enough to be used in sensitive applications, such as wound care and food sanitation. Understanding its chemical structure and properties is key to appreciating its versatility and effectiveness.
HOCl is formed when chlorine dissolves in water, undergoing a reaction that produces both hypochlorous acid and hydrochloric acid. This chemical reaction is fundamental to its widespread use in disinfection processes. For instance, in swimming pools, hypochlorous acid is the active ingredient in chlorine-based sanitizers that keep the water safe for use. Its ability to eliminate harmful microorganisms has made it a cornerstone of modern hygiene practices.
Beyond its industrial applications, hypochlorous acid has gained attention in recent years for its eco-friendly and biocompatible nature. Unlike many chemical disinfectants, HOCl breaks down into harmless byproducts, making it a sustainable choice for a variety of uses. Whether you're a scientist, a healthcare professional, or simply someone curious about chemistry, understanding the formula for hypochlorous acid provides valuable insights into its applications and benefits.
Read also:What Are Examples A Comprehensive Guide To Understanding And Using Examples Effectively
Table of Contents
- What is Hypochlorous Acid and Why is it Important?
- The Chemical Properties of HOCl: How Does it Work?
- What Are the Applications of Hypochlorous Acid?
- How Does Hypochlorous Acid Impact the Environment?
- How is Hypochlorous Acid Produced?
- What Are the Safety Concerns with HOCl?
- What Does the Future Hold for Hypochlorous Acid?
- Frequently Asked Questions About Hypochlorous Acid
What is Hypochlorous Acid and Why is it Important?
Hypochlorous acid, with the chemical formula HOCl, is a weak acid that forms when chlorine dissolves in water. This compound is a naturally occurring substance that the human immune system produces to fight infections. In fact, white blood cells generate hypochlorous acid to destroy bacteria, viruses, and other pathogens. This biological function underscores its importance in maintaining health and hygiene.
From a chemical perspective, HOCl is a powerful oxidizing agent. It works by disrupting the cell walls of microorganisms, leading to their destruction. This property makes it an invaluable tool in disinfection and sanitation. For example, hypochlorous acid is widely used in hospitals to sterilize equipment and surfaces, ensuring a safe environment for patients and healthcare workers alike.
Another reason for its significance is its eco-friendly nature. Unlike many chemical disinfectants that leave harmful residues, hypochlorous acid breaks down into water and salt, making it safe for the environment. This characteristic has led to its adoption in industries ranging from agriculture to food processing, where sustainability is a growing concern.
Why is the Formula for Hypochlorous Acid So Unique?
The formula for hypochlorous acid, HOCl, is deceptively simple, yet it holds the key to its remarkable properties. Unlike stronger acids, HOCl is weakly acidic, which allows it to be effective without causing damage to surfaces or living tissues. This balance between potency and safety is what makes it so unique.
Additionally, HOCl's molecular structure enables it to penetrate microbial cell walls easily. Once inside, it oxidizes critical components, effectively neutralizing the pathogen. This mechanism of action is highly efficient, requiring only small concentrations of the acid to achieve significant results.
Moreover, the formula for hypochlorous acid highlights its versatility. It can exist in different forms, such as a liquid solution or a gas, depending on the conditions. This adaptability allows it to be used in a wide range of applications, from industrial cleaning to personal care products.
Read also:Barry Weiss The Visionary Leader Transforming Industries
How Does Hypochlorous Acid Compare to Other Disinfectants?
When comparing hypochlorous acid to other disinfectants, several factors set it apart. For one, its effectiveness is unmatched. HOCl can kill a broad spectrum of microorganisms, including bacteria, viruses, fungi, and spores, often in a matter of seconds. This rapid action makes it ideal for situations where time is of the essence, such as in emergency healthcare settings.
Another advantage is its safety profile. Unlike bleach or hydrogen peroxide, which can be corrosive or irritating, hypochlorous acid is gentle on skin and surfaces. This makes it suitable for applications like wound care, where patient comfort is paramount. Additionally, its non-toxic nature ensures that it can be used in food processing without posing health risks.
Finally, hypochlorous acid is more environmentally friendly than many alternatives. It decomposes into harmless substances, reducing the risk of pollution. This aligns with the growing demand for sustainable solutions in various industries, making HOCl a preferred choice for those seeking both efficacy and eco-consciousness.
The Chemical Properties of HOCl: How Does it Work?
To fully appreciate the formula for hypochlorous acid, it's essential to understand its chemical properties. HOCl is a weak acid, meaning it partially dissociates in water to form hydrogen ions (H⁺) and hypochlorite ions (OCl⁻). This dissociation is crucial to its function as a disinfectant, as both forms contribute to its antimicrobial activity.
One of the most notable properties of hypochlorous acid is its oxidizing power. It readily donates oxygen atoms to other molecules, disrupting their structure and function. This oxidative stress is particularly damaging to microorganisms, as it targets essential components like proteins, lipids, and DNA. As a result, pathogens are unable to survive or reproduce.
Another key property is its stability. While HOCl is effective in its active form, it is also relatively unstable, breaking down quickly in the presence of light, heat, or organic matter. This instability ensures that it does not persist in the environment, reducing the risk of long-term contamination. However, it also means that solutions of hypochlorous acid must be used promptly to maintain their efficacy.
What Makes HOCl So Effective Against Pathogens?
The effectiveness of hypochlorous acid against pathogens can be attributed to its ability to penetrate microbial cell walls. Once inside, it targets critical cellular components, such as enzymes and nucleic acids, disrupting their function. This multi-faceted attack ensures that microorganisms are neutralized quickly and efficiently.
Furthermore, HOCl's dual action as both an acid and an oxidizing agent enhances its potency. The acidic environment it creates can denature proteins and disrupt cellular processes, while its oxidizing power damages structural components. Together, these mechanisms create a synergistic effect that maximizes its disinfectant capabilities.
Finally, the formula for hypochlorous acid allows it to exist in equilibrium with its hypochlorite ion (OCl⁻). This dynamic balance ensures that it remains active in a variety of conditions, adapting to changes in pH and temperature. This adaptability is a key factor in its widespread use across different applications.
What Are the Applications of Hypochlorous Acid?
The applications of hypochlorous acid are vast and varied, thanks to its unique combination of properties. In healthcare, HOCl is used for wound care, as it promotes healing while preventing infection. Its gentle nature makes it suitable for use on sensitive skin, and its rapid action ensures that pathogens are eliminated quickly.
In the food industry, hypochlorous acid is a preferred sanitizing agent. It is used to clean equipment, surfaces, and even fresh produce, ensuring that food is safe for consumption. Its non-toxic nature means that it can be applied directly to food without leaving harmful residues, making it an ideal choice for food safety protocols.
Other applications include water treatment, where HOCl is used to disinfect drinking water and swimming pools. Its ability to kill a wide range of microorganisms ensures that water remains safe for use. Additionally, it is employed in agriculture to sanitize crops and prevent the spread of plant diseases, contributing to higher yields and better quality produce.
How is Hypochlorous Acid Used in Everyday Life?
In everyday life, hypochlorous acid is found in a variety of household products. For instance, it is a key ingredient in many disinfectant sprays and wipes, offering a convenient way to sanitize surfaces. Its effectiveness against common germs makes it a popular choice for maintaining a clean and healthy home environment.
HOCl is also used in personal care products, such as mouthwashes and skin cleansers. Its antimicrobial properties help to reduce the risk of infections, while its gentle nature ensures that it does not irritate sensitive tissues. This dual benefit has made it a staple in many households, particularly for families with young children or individuals with compromised immune systems.
Finally, hypochlorous acid is gaining traction in the pet care industry. It is used to clean pet bedding, toys, and grooming tools, ensuring that pets remain healthy and free from infections. Its non-toxic nature makes it safe for use around animals, providing peace of mind for pet owners.
How Does Hypochlorous Acid Impact the Environment?
One of the most appealing aspects of hypochlorous acid is its minimal environmental impact. Unlike many chemical disinfectants, HOCl breaks down into harmless substances, such as water and salt, after use. This ensures that it does not contribute to pollution or harm ecosystems, making it a sustainable choice for various applications.
Furthermore, its biodegradability aligns with the growing demand for eco-friendly solutions. In industries like agriculture and food processing, where large volumes of disinfectants are used, the environmental benefits of hypochlorous acid are particularly significant. Its use reduces the risk of contaminating soil and water, preserving natural resources for future generations.
However, it's important to note that the production of hypochlorous acid must also be sustainable. While the formula for hypochlorous acid itself is environmentally friendly, the methods used to generate it can vary. Ensuring that production processes are energy-efficient and minimize waste is essential to maximizing its overall sustainability.
What Are the Challenges of Using Hypochlorous Acid in the Environment?
Despite its many benefits, there are challenges associated with using hypochlorous acid in the environment. One issue is its instability, which can limit its shelf life and effectiveness. Solutions of HOCl must be stored in dark, cool conditions to prevent degradation, which can be inconvenient for some users.
Another challenge is the potential for misuse. While hypochlorous acid is generally safe, improper handling or excessive use can lead to unintended consequences. For example, overuse in water treatment systems may result in the formation of harmful byproducts, such as chloramines. Educating users about proper application techniques is crucial to mitigating these risks.
Finally, the cost of producing high-quality hypochlorous acid can be a barrier for some industries. While its benefits outweigh the costs in many cases, finding cost-effective production methods is essential to making it accessible to a wider audience. Ongoing research and innovation in this area are likely to address these challenges in the future.
How is Hypochlorous Acid Produced?
The production of hypochlorous acid typically involves the electrolysis of a saltwater solution. This process generates chlorine gas, which is then dissolved in water to form HOCl. While this method is widely used, it requires careful control to ensure the purity and stability of the final product.
Another method involves the direct reaction of chlorine with water. This approach is simpler but less precise, as it can result in the formation of byproducts like hydrochloric acid. To minimize these byproducts, manufacturers often use advanced technologies, such as membrane electrolysis, to produce high-quality hypochlorous acid.
Recent advancements in production methods have focused on improving efficiency and sustainability. For example, some companies are exploring the use of renewable energy sources to power the electrolysis process. This not only reduces the environmental impact but also lowers production costs, making hypochlorous acid more accessible to a broader range of industries.
What Are the Safety Concerns with HOCl?
While hypochlorous acid is generally safe, there are some safety concerns to be aware of. For one, its instability means that it can degrade quickly if not stored properly. Exposure to light, heat, or organic matter can reduce its effectiveness, so users must take care to follow storage guidelines.
Another concern is the potential for skin or eye irritation if used in high concentrations. While HOCl is gentle compared to other disinfectants, excessive exposure can still cause discomfort. To mitigate this risk, it's important to use diluted solutions and follow recommended application guidelines.
Finally, the production of hypochlorous acid must be
Darla In The Little Rascals: A Nostalgic Dive Into A Beloved Character
What Is The Salt Trick For ED? Discover How It Can Help
Discover Your Destiny: March 17th Horoscope Insights
HClO Hypochlorous Acid Molecule Model and Chemical Formula Stock Vector

MJ6001 Hypochlorous Acid bactericide RAYMOND